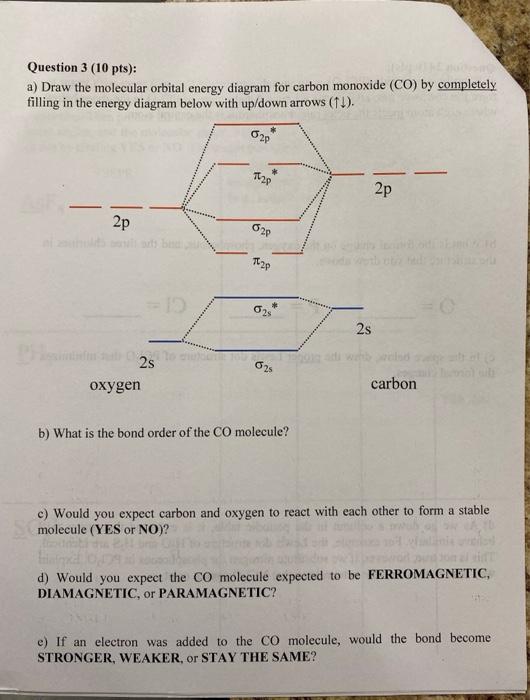
how to draw molecular orbital diagram of co
Hence Lewis Structure is also commonly called Electron Dot Structure. Note the bonds that are forming N-H C-C and the bonds that are breaking CH CO.
How Co Forms According To Molecular Orbital Theory Please Clarify The Concept Of Hybridization Involved In This According To Molecular Orbital Theory Quora
The hyphens represent.
. Lewis structure of carbon monoxide is drawn below. How It Works. In a Lewis structure these six dots are arranged so that an atom has two lone pairs and two single electrons.
O 2 is the most abundant molecular ion in the ionosphere of Venus. Thus we can see that there are six electrons that need to be apportioned to Crystal Field Diagrams. For example if you were to draw out the following equation for a reaction between 2 hydrogen and 2 bromine.
The smaller bodies ie debris are composed of molecular matter. Two elements in Period 4 are adjacent to one another in the periodic table. As explanation the fast exothermic reaction O CO 2 O 2 CO where the O ions are released from dissociative ionization of CO 2 is proposed However this mechanism cannot play a role in media where CO 2 is absent or has a low concentration such as in the.
This diagram below shows the reaction between LDA and the ketone. SATVI Immunology Department of Pathology. The skeletal formula or line-angle formula or shorthand formula of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecules bonding and some details of its molecular geometryA skeletal formula shows the skeletal structure or skeleton of a molecule which is composed of the skeletal atoms that make up the.
Pyridine and is the first paper in the literature describing the reaction of alcohols. Ar4s 0 3d 6. Lewis Structure is the name given to such a skeletal diagram where we use the symbols of the atoms and use dots to represent the valence shell electrons.
Then the next electron leaves the 3d orbital and the configuration becomes. In molecular orbital theory when the bonding takes place the atomic orbitals that take. According to the property of entropy energy always seeks the lowest possible state of order.
This can occur in two ways. Let us proceed to draw the most appropriate LS diagram of CO32- ion. This is the paper rationalizing the differing stereochemistries of the reaction of alcohols with SOCl 2 in the presenceabsence of base eg.
Cram would later on receive the Nobel Prize in Chemistry in 1987 for his work on molecular host-guest chemistry. According to Kossel and Lewis atoms combine together in order to complete their respective octets so as to acquire the stable inert gas configuration. Note that each shell lies further and further out from the nucleus of the atom.
Group 2 elements contain 2 valence electrons only in s-orbital. Explain the formation of a chemical bond. The electrons will seek to populate the lowest orbital shells available.
A Lewis structure is based on the concept of the octet rule in which atoms share electrons so that each atom has eight electrons in its outer shellAs an example an oxygen atom has six electrons in its outer shell. TB vaccines and immunology. The enolate that is formed has a resonance isomer where the negative charge is on the carbon.
Department of Molecular Cell Biology Science Faculty. NCERT Solutions Class 11 Chemistry Chemistry Lab Manual Chemistry Sample Papers. Medical Biochemistry Structural Biology Department of Integrative Biomedical Sciences.
Moreover the Sun is a fundamentally different type of body where it is a ball of plasma powered by nuclear fusion in its core. The first two to go are from the 4s orbital and Cobalt becomesAr4s 0 3d 7. Given molecule is CO.
H 2 g Br 2 g --- 2 HBrg you would get. Protein biochemistry angiotensin. Single double and triple bonds have different bond energies so be sure to draw your diagram with the correct bonds between elements.
When observing Cobalt 3 we know that Cobalt must lose three electrons. Oncogenic viruses cancer biology. If we instead measure the Solar System in terms of angular momentum then most of it lies in the orbital motions of the planets.
In molecular orbital theory when the bonding takes place the atomic orbitals that take. Consult a diagram of electron orbital shells. NCERT TEXTBOOK QUESTIONS SOLVED.
Count the Total Number of Valence Electrons. H-H Br-Br --- 2 H-Br. Group 3 elements contain 2 electrons Group 3 elements contain 2 electrons Q.

What Is The Molecular Orbital Energy Diagram Of Co Quora
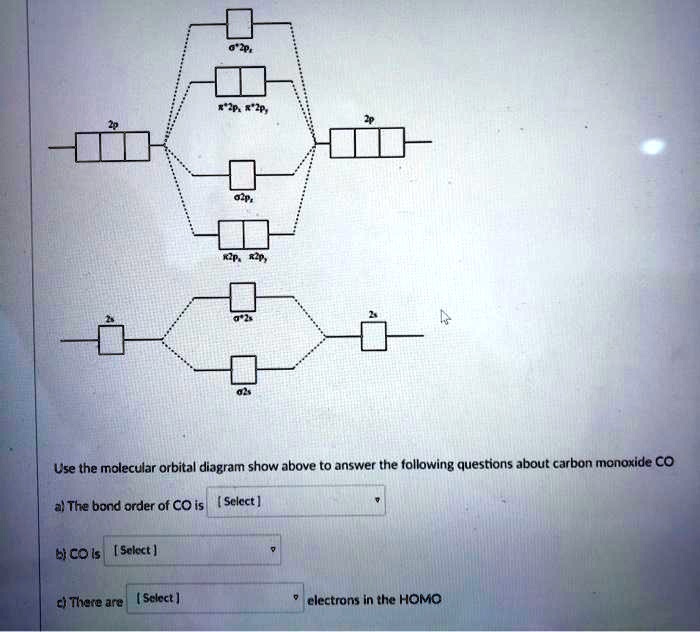
Solved Use The Molecular Orbital Diagram Show Above To Answer The Following Questions About Carbon Monoxide Co The Bond Order Of Co Is Sclect Bicois Select Ci There Are Sclect
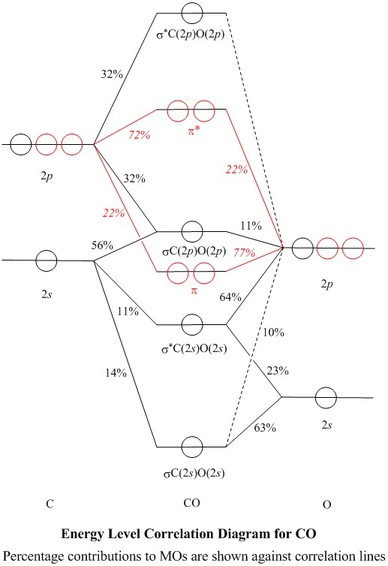
Molecular Orbitals For Carbon Monoxide

Draw The Molecular Orbital Diagram For Co Based On Your Diagram Why Does Co Always Bond Through The Carbon And Not The Oxygen Atom Homework Study Com

Solved 1 Draw The Molecular Orbital Energy Diagram For Chegg Com
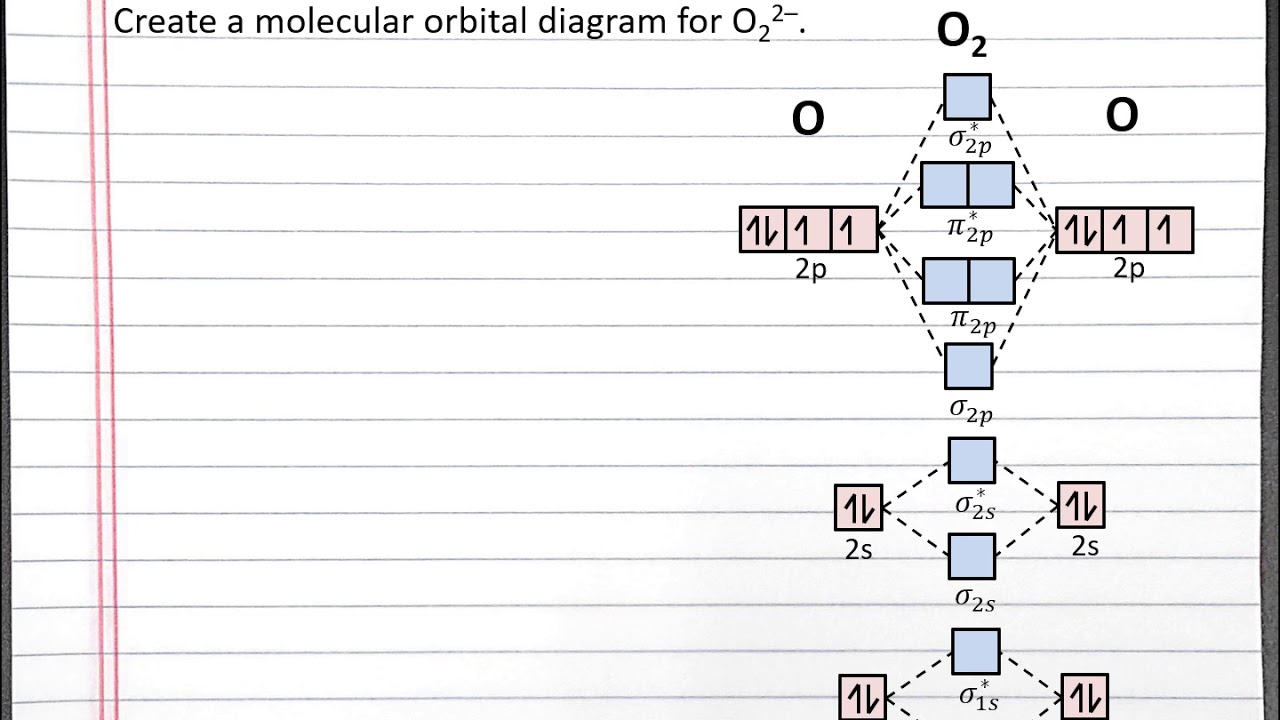
Chem 101 Creating A Molecular Orbital Diagram For A Diatomic Ion In The Second Row With Aleks Youtube

Left Simplified Mo Diagram Of Co With Electronic Occupancy In The Download Scientific Diagram
What Is The Bond Order Of Co Quora

Draw The Molecular Orbitals For Co In Order Of Energy And Fill Them With The Appropriate Number Of Electrons Label The Orbitals The Best You Can As Sigma Or Pi And As

Draw The Molecular Orbital Diagrams For The Following Diatomic Molecules Polyatomic Ions Indicate Their Bond Orders And Rank Them In Order Of Increasing Bond Strength A Cn B Co C F 2 D N 2
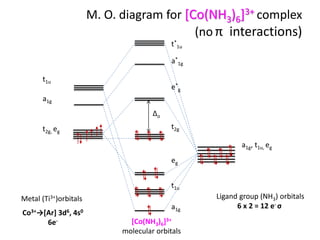
Molecular Orbitals Diagrams Of Co Nh3 6 3
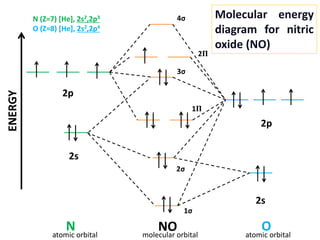
Molecular Orbital Diagram Of Co And No

What Would Be The Mo Diagram For Co F 2 No Of O 2 And Ne 2 Molecules Calculate The Bond Order In Each And Predict The Type Of Magnetism Found If Any Homework Study Com
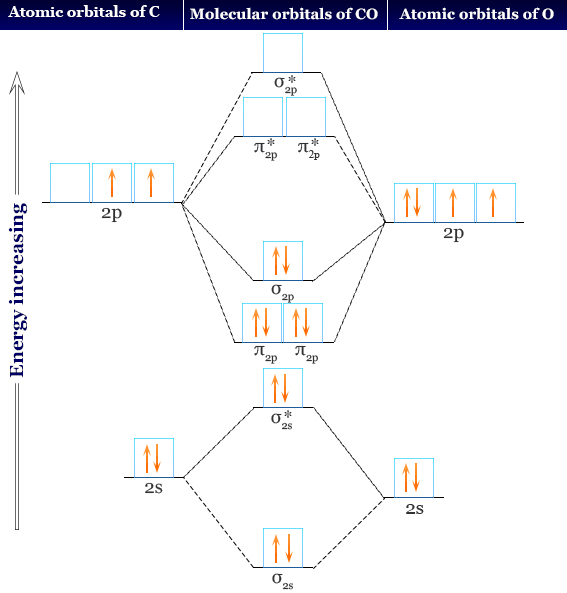
Carbon Monoxide Facts Bonding Properties Uses

Mo Diagram Overview How To Draw Mo Diagram And Solved Example Along With Faqs

Can I Predict The Preferred Resonance Structure Of Carbon Monoxide From Its Molecular Orbital Scheme Chemistry Stack Exchange

Molecular Orbital Diagram Of The Co Molecule Excluding 1s Atomic Download Scientific Diagram